Abstract
INTRODUCTION: Immature myeloid (IM) cells have been shown to induce immune dysfunction(s) in non-Hodgkin's lymphoma (NHL) patients. Myeloid derived suppressor cells (MDSCs) are a subset of IM cells that play a key role in immune suppression associated with malignancies. In solid tumors, MDSCs have been implicated in promoting angiogenesis, tumor cell invasion and metastasis. The presence of MDSCs may correlate with decreased efficacy of immunotherapies. MDSCs have been associated with reduced survival in solid tumors. We aimed at identifying the patterns of immature myeloid cell subsets including MDSCs in DLBCL patients, pre and post-standard R-CHOP or R-EPOCH therapy.
METHODS: Peripheral blood was collected on 31 patients with de novo diffuse large B cell lymphoma on an IRB approved study before and after frontline chemo-immunotherapy and at the time of relapse. Clinical variables at diagnosis (age, performance status, stage of the disease, number of extra nodal lesions) were obtained to calculate prognostic indices (IPI and R-IPI). Cell of origin was determined by IHC using the Hans algorithm. Response to therapy was assessed as per the Lugano criteria for response and assessment in lymphoma. Peripheral distribution of myeloid cells (CD11b+ CD33+), IM cells (HLA-DR-/low IL-4R+) and immunosuppressive IM cell subsets [CD14+ monocytic (m) and CD15+ granulocytic (g) myeloid-derived suppressor cells (MDSCs)] in fresh whole blood samples were established by flow cytometry.
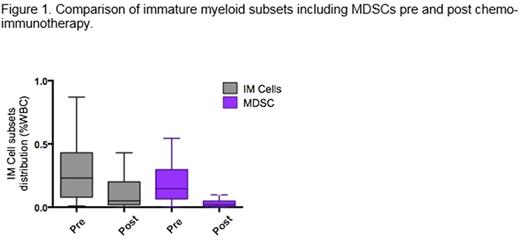
RESULTS: Median age was 65 years (range 24-88). Twenty-two (70.9%) patients had stage III/IV disease. Thirteen patients (41.9%) had GCB subtype, 16 patients (51.6%) had non-GCB subtype and cell of origin could not be determined in 2 patients (6.5%). Nineteen patients (61.3%) had IPI scores 0-2 and 12 (38.7%) patients had IPI scores of 3-5. Twenty-two patients (70.9%) received R-CHOP or R-mini CHOP, and 9 patients (29%) received R-EPOCH. The end of therapy ORR and CR were 100% and 96.7% respectively. One patient had a PR. Median follow up was 17.5 months. Two patients (6.4%) subsequently progressed. Both are alive after subsequent therapy. The overall survival is 100%. Prior to treatment initiation, patients presented with 0.23±0.05% circulating IM cells, of which 51.6±5.6% were mMDSC and 11.2±4.4% were gMDSC. No correlation between IM cell distribution (including MDSCs) and IPI or disease stage was observed. A trend was observed towards higher mMDSCs in GCB subtype compared to non-GCB subtype (3.49±1.4% vs. 1.44±0.7% of total myeloid cells; p=0.086). Following treatment with chemo-immunotherapy, overall distribution of circulating IM cells decreased to 0.05±0.04% of total WBC (p=0.012 compared with baseline), 31.2±5.6% of which were mMDSC and 21.3±3.7% were gMDSC. Normalization of IM cell distribution was independent of chemo-immunotherapy regimen or IPI. Compared to early stage disease (I/II), patients with advanced stage disease (stage III/IV) tended to retain more IM cells at the end of therapy (0.06±0.03 vs. 0.03±0.03% of total WBC; p=0.069), a significant proportion of which are mMDSC (0.36±0.25 vs. 0.25±0.07% of total myeloid cells; p=0.032). The two patients who relapsed after initial therapy displayed high proportions of MDSCs among total IM cells throughout the course of their disease compared with non-relapsing patients: at baseline (84.8-97.7% vs. 62.2±4.9% of total IM cells), post treatment (87.7-95.7% vs. 52.5±4.4% of total IM cells). This trend was conserved upon relapse with MDCSs representing 91-93% of total IM cells.
CONCLUSIONS: To the best of our knowledge, this is the first study to prospectively examine immature myeloid cells and MDSCs in DLBCL at diagnosis, end of therapy and at the time of relapse. Circulating MDSCs, are associated with an active state of DLBCL. Response to chemo-immunotherapy was associated with a dramatic decrease of circulating IM cells, including MDSCs. Patients with advanced stage disease retained a higher titer of circulating mMDSC at end of therapy. For patients who relapsed, a disproportionate abundance of MDSCs (>85% of total IM cells) were seen at diagnosis, post treatment and at the time of relapse. Taken together, MDSCs may represent a potential biomarker and therapeutic target for relapsed and refractory DLBCL patients.
Park: Teva: Consultancy, Research Funding; Takeda: Research Funding; Cornerstone Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gilead: Speakers Bureau; Seattle Genetics: Research Funding. Ghosh: Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Speakers Bureau; Jassen: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal